Immune checkpoint blockade has generated dramatic responses in certain types of human tumors. However, the response of breast tumors has been largely limited. We have previously demonstrated that the residence of breast cancer cells in the epithelial or mesenchymal phenotypic states can itself be used as an important determinant of the success or failure of immune checkpoint blockade. The Dongre Lab is focused on understanding the mechanisms by which the EMT program drives resistance of breast tumors to anti-tumor immunity. We work with novel models and cutting-edge techniques right at the interface of cancer biology and immunology.
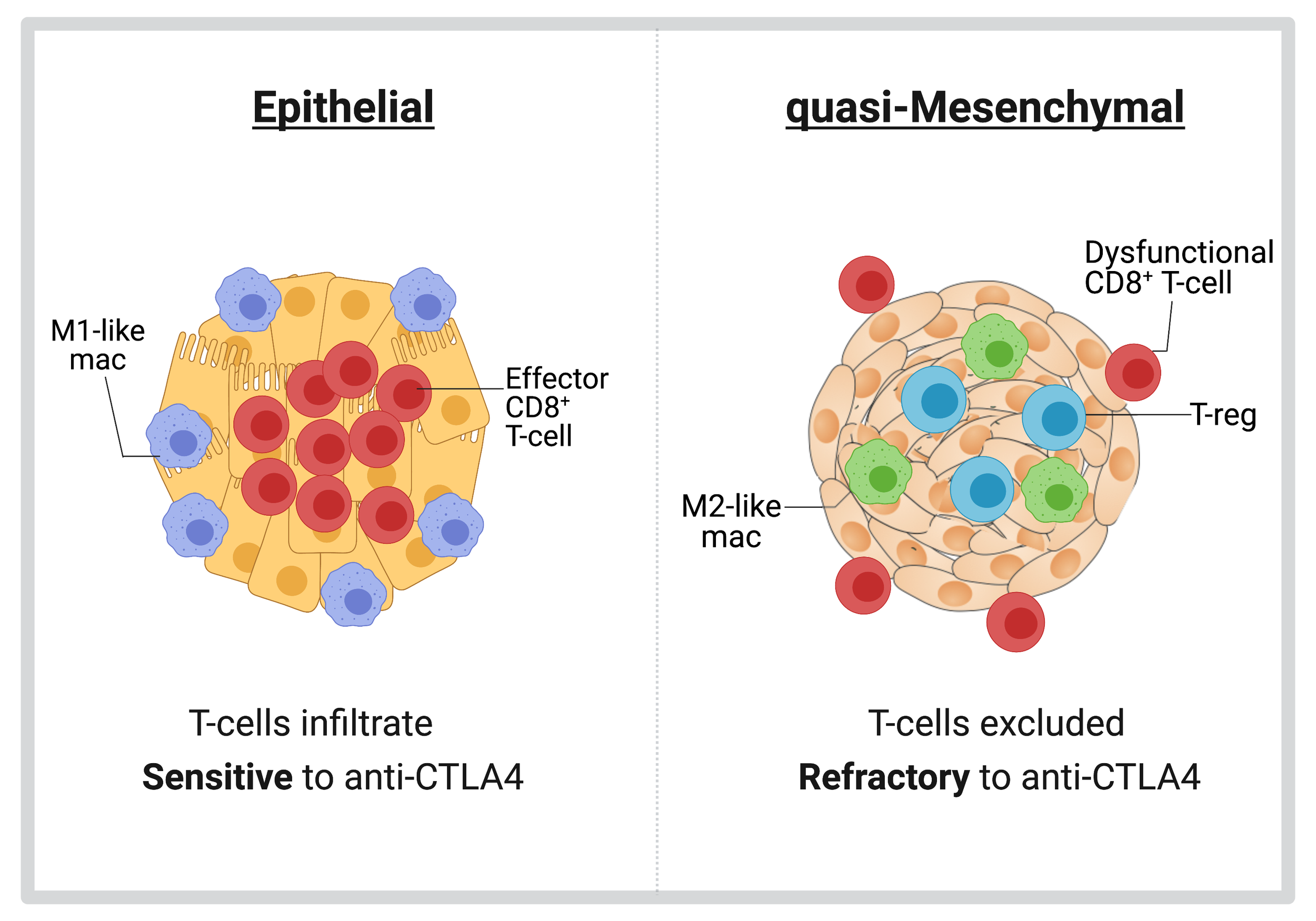
Differential susceptibility of epithelial and quasi-mesenchymal tumors to anti-tumor immunity.
By using novel, preclinical murine models of epithelial and quasi-mesenchymal breast tumors, we have observed that these two tumor types assemble dramatically different tumor microenvironments and differ in their susceptibility to anti-CTLA4 therapy. Specifically, epithelial tumors are infiltrated by CD8+ T-cells and are sensitive to anti-CTLA4 therapy. In sharp contrast, quasi-mesenchymal tumors recruit immunosuppressive Tregs and M2-like macrophages and exclude exhausted CD8+T-cells to the periphery. Importantly, quasi-mesenchymal tumors are resistant to anti-CTLA4 therapy. (Cancer Research 2017). We are actively investigating the underlying mechanisms that dictate these differential responses of epithelial and quasi-mesenchymal tumors to multiple forms of immune checkpoint blockade therapy. To this end, we are using our preclinical mouse models in conjunction with CRISPR/Cas9 editing, flow cytometry, and imaging techniques.
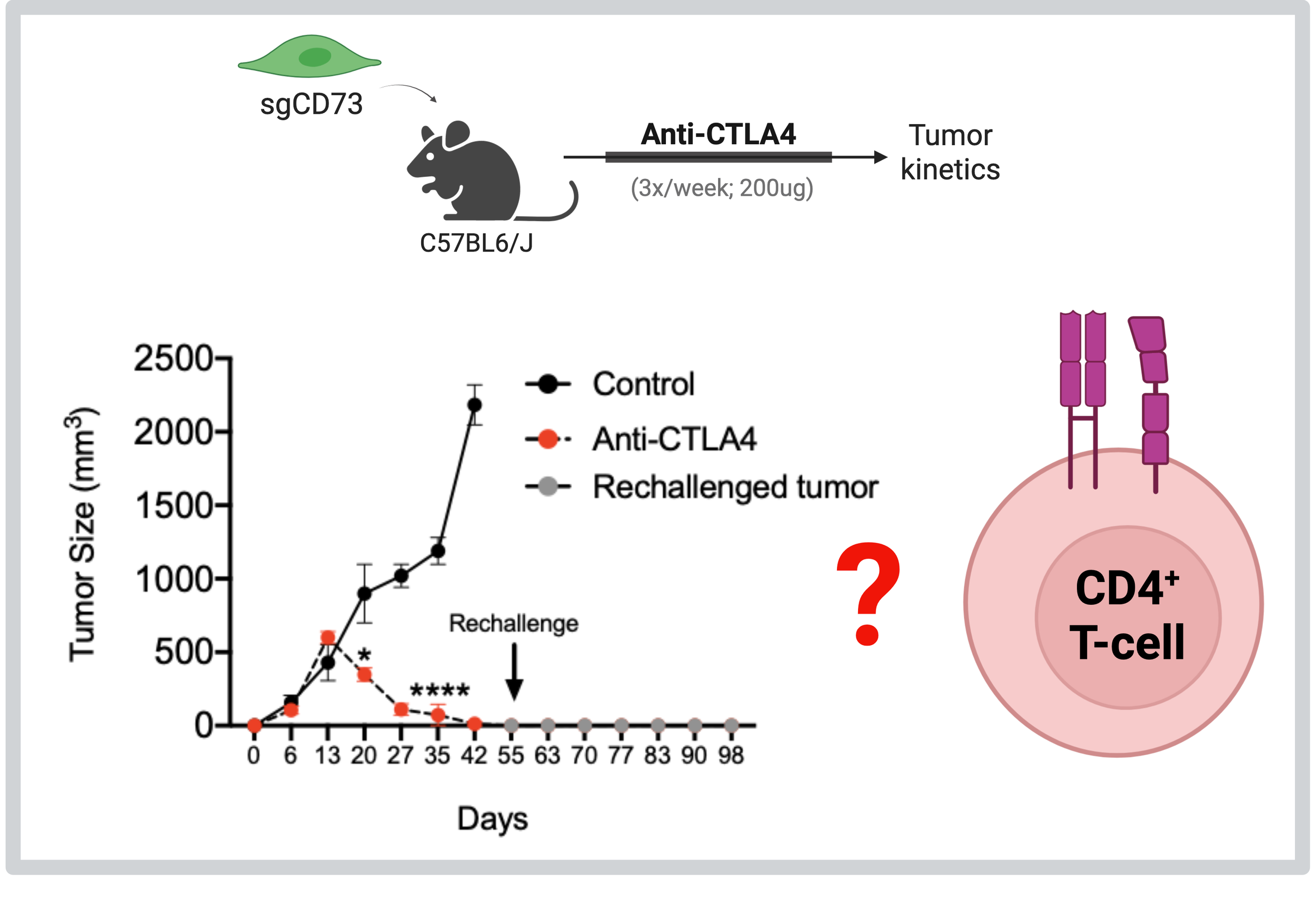
Targeting CD73 sensitizes quasi-mesenchymal tumors to anti-CTLA4 therapy via CD4+ T-cells
By undertaking a series of transcriptomic approaches, we have found that quasi-mesenchymal carcinoma cells express multiple cell-intrinsic immunosuppressive factors relative to their epithelial counterparts. Most strikingly, abrogation of CD73 from qM cancer cells completely sensitizes these refractory tumors to anti-CTLA4 immune checkpoint blockade therapy (Cancer Discovery 2021). Moreover, this sensitization is dependent on CD4+ T-cells (bioRxiv 2025). We are delving into various mechanisms utilized by CD4+ T-cells in order to eliminate CD73-deficient quasi-mesenchymal tumors in response to anti-CTLA4 therapy. We are also determining the response of CD73-deficient quasi-mesenchymal tumors to other forms of checkpoint inhibition.
Cross-protective effects of quasi-mesenchymal cancer cells in heterogeneous breast tumors
In mixed tumors comprised of both epithelial and quasi-mesenchymal cancer cells, minority populations (10%) of quasi-mesenchymal cells can cross-protect the vast majority (90%) of their epithelial neighbors residing in the same tumor from immune attack (Cancer Research 2017). The mechanisms underlying these cross-protective effects remain unclear. Understanding these mechanisms is particularly important as most human breast cancers contain minority populations of mesenchymal cells which can dictate the response of the tumor as a whole to immune checkpoint blockade. We are using spatio-temporal approaches to understand how quasi-mesenchymal carcinoma cells exert cross-protective effects in mixed tumors.
Perturbing epithelial-mesenchymal plasticity to make breast tumors vulnerable to immunotherapy
The EMT program is a reversible process in which carcinoma cells residing in a mesenchymal state can lapse back into an epithelial state by activating the reverse process of mesenchymal-to-epithelial transition. However, the degree to which the EMT program must be reversed in order to sensitize tumors to ICB therapies remains to be determined. Indeed, the residence of carcinoma cells in the mesenchymal state is maintained by cytokines such as TGF-β1, or EMT Transcription factors themselves. Strikingly, TGF-β1 is highly immunosuppressive and can directly blunt the cytolytic activities of T-cells. Thus, perturbing plasticity would represent an attractive strategy to not only cellular plasticity, but concurrently sensitize them to anti-tumor immunity. We are using our pre-clinical, immunocompetent murine models of mesenchymal tumors in conjunction with CRISPR/Cas9 and immunological approaches to address this question.
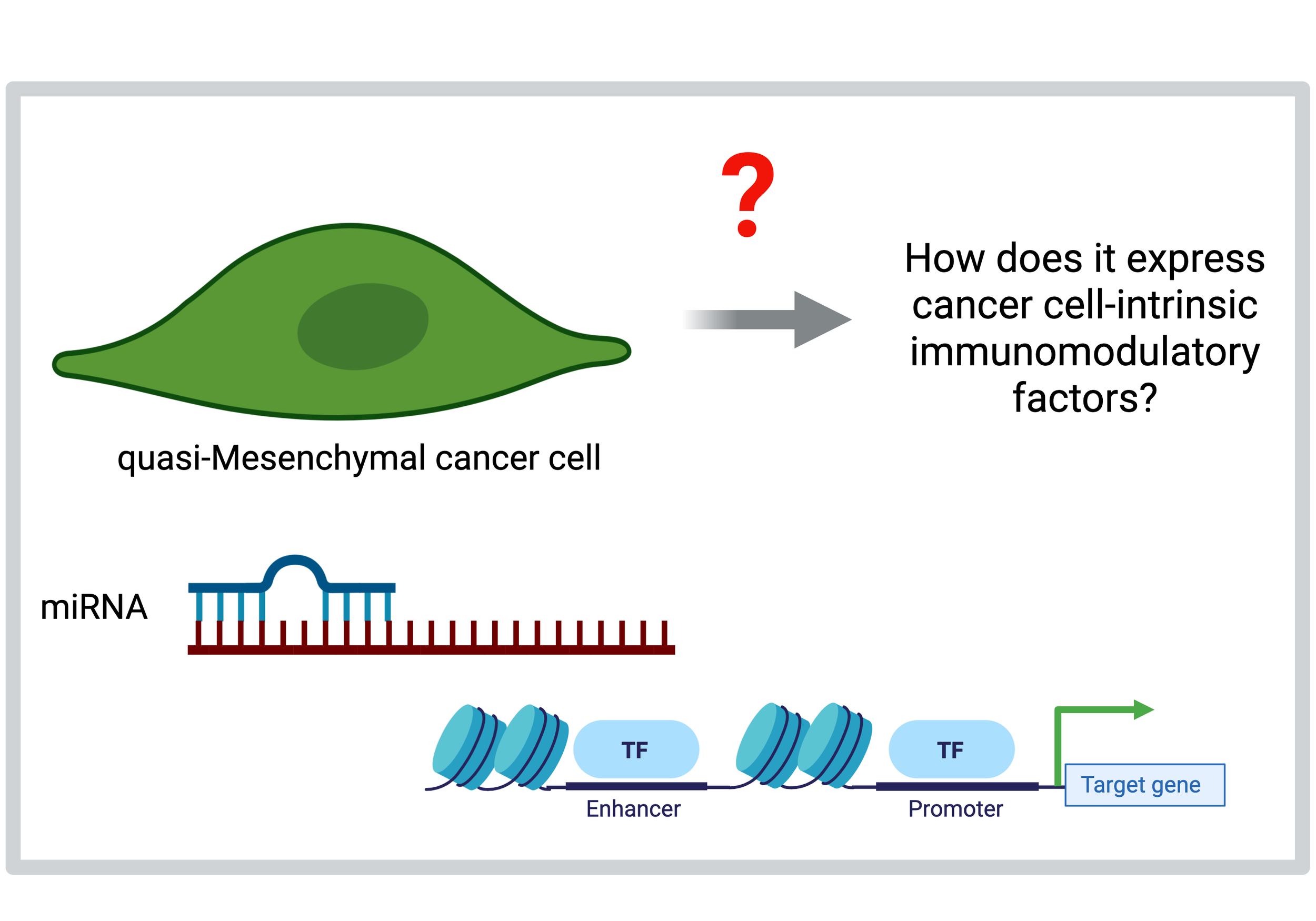
Regulation of cell-intrinsic immuno-modulatory factors in breast cancer cells undergoing an EMT
We have previously determined that breast cancer cells undergoing an EMT express multiple cell-intrinsic immunomodulatory factors (Cancer Discovery 2021). However, the precise molecular mechanisms that regulate the expression of these factors is unknown. Moreover, the kinetics of expression of cancer cell-intrinsic immunomodulatory factors as cells undergo an EMT remains to be determined. In a collaborative effort with the Sethupathy Lab, we are determining the genomic landscape of cancer cells undergoing an EMT to identify and subsequently target genetic and epigenetic drivers of immunotherapy response.